Researchers working with Jonathan Barnes, assistant professor in the Department of Chemistry, have recently shown how molecules with interlocking ring architectures can be functionalized and incorporated into three-dimensional polymer networks and materials. First author Mark Nosiglia, a graduate student in Barnes’ lab, led the new work, which builds on the team’s previous efforts to streamline the synthesis of mechanically interlocked molecules. The results were published May 26 in the Journal of the American Chemical Society.
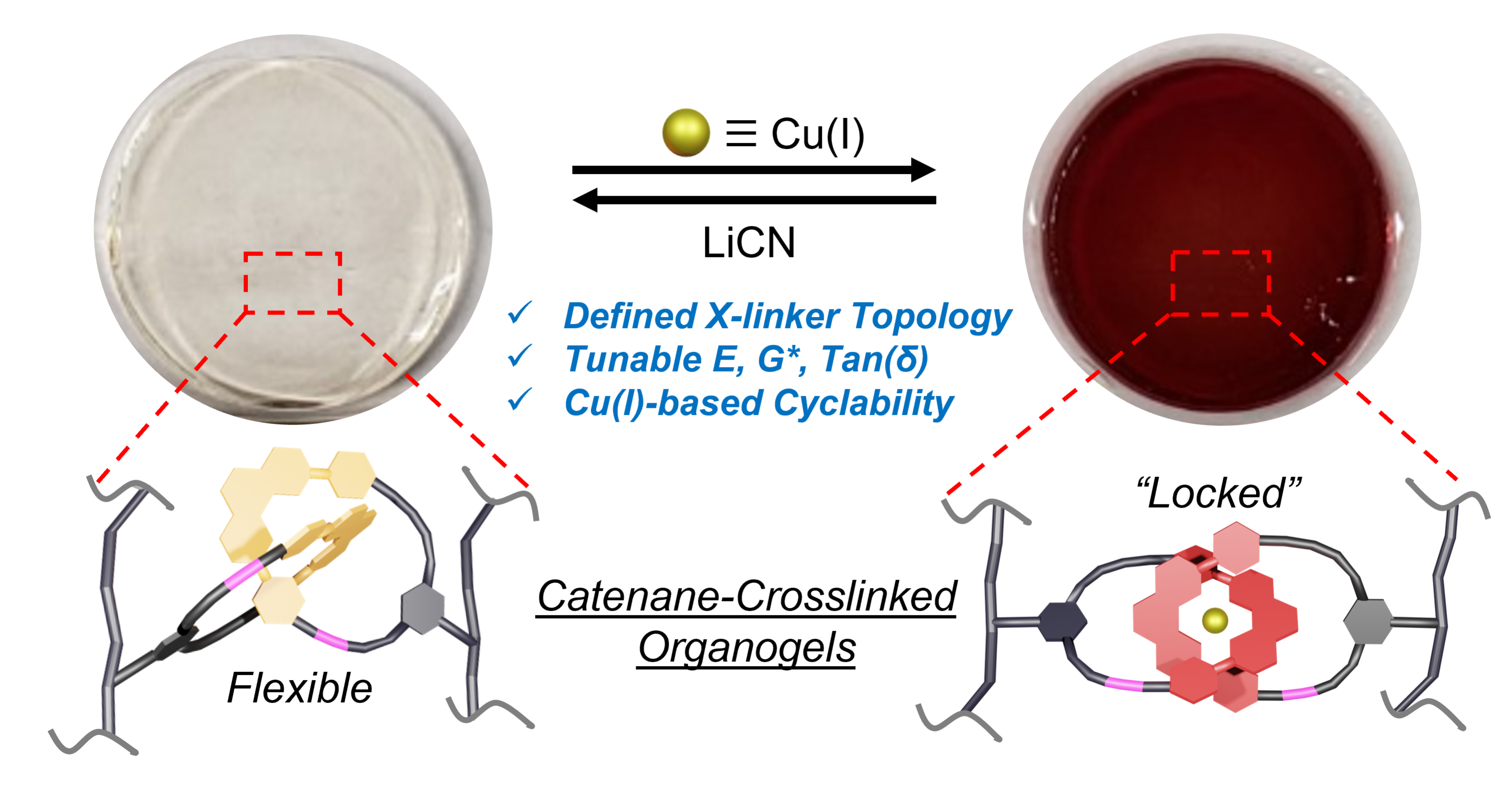
After streamlining and improving the efficiency of their synthesis methods, Barnes and Nosiglia sought to tune the stiffness, elasticity, and force dissipation properties of materials by integrating catenane-based crosslinkers into the network that makes up the material. Catenanes are mechanically interlocked molecules consisting of two or more rings, which allows them – and the material in which they’re incorporated – enough freedom of movement to do things like rotate, stretch, and compress when subjected to external forces.
Barnes and Nosiglia found that by adding a metal to, or “metalating,” the catenanes, they could fix the rings into a particular conformation, causing the entire gel material to become more rigid and less elastic.
“By incorporating molecular chains that can be ‘locked’ into the network, it should be possible to tune the properties of materials,” Barnes explained. “Potential applications may include using the molecular ring architectures in rubber-like materials and plastics to improve stretchability and their ability to dissipate forces, including impacts, stretching, and flexing.”
Up next, Barnes, Nosiglia, and their collaborators are focusing on producing their 3D networked materials at large enough scales to fully explore and test their mechanical properties. Such scaling up will be an essential part of the team’s future research endeavors.
Read the full paper, “Metalation/Demetalation as a Postgelation Strategy To Tune the Mechanical Properties of Catenane-Crosslinked Gels.”