New research from the Department of Earth and Planetary Sciences uses a previously unstudied mineral to measure rates of volcanic activity.
Volcanic eruptions are some of the briefest phenomena studied by geoscientists. In a field that deals with billion-year timescales, how do scientists study events lasting only days or weeks? One tool used by geochemists is the rate of chemical diffusion in certain minerals. The mixing of chemical elements can tell scientists how much time passed between precursor event and eruption.
New research from Michael Krawczynski, assistant professor of earth and planetary sciences, with lead author Kelsey Prissel, a graduate student in Krawczynski’s experimental geochemistry lab, addresses a major gap in geochemical diffusion data used to study volcanic activity. Though diffusion rates in minerals have long been studied, similar data for the common mineral ilmenite have been entirely absent from published research until now. The work was published in Contributions to Mineralogy and Petrology.

“Diffusion rates in ilmenite have never been quantified,” Prissel noted. “That’s odd given how common it is and how well studied the similar mineral magnetite has been since the 1970s. We know so much about diffusion in magnetite – there’s decades of work – but there was not one study we could find on ilmenite.”
Unlike other common rock-forming minerals, in which diffusion might take decades to millions of years, this study shows that ilmenite has a much faster diffusion rate of weeks to months. That offers geoscientists a new tool to look at volcanic events happening on a shorter timescale.
The geochemical data developed in this study as a collaboration between Washington University in St. Louis and Case Western Reserve University can be used to complement geophysical insights into volcanic activity. Physical methods for monitoring volcanoes typically entail looking at the current state of the volcano – for example, what’s happening now in seismology or surface deformation? Geochemistry offers a way to look back in time based on the rate of known reactions, opening a window to past volcanic events and setting a timer on the process leading up to eruption.
“We can’t predict when a volcano might erupt with geochemistry because we have to wait until after the eruption to see that geochemistry,” Krawczynski said. “However, we can analyze the eruptive products and use geospeedometers, like the one calibrated in this study, to pinpoint when the change that led to the eruption happened.”
Geospeedometry, which Krawczynski described as “the radar gun of geology,” measures the timing of thermal events in geologic materials. In this case, the diffusion rates Krawczynski and Prissel determined for the mineral ilmenite can be used to estimate the time between magmatic perturbation and volcanic eruption on a timescale of hours to months.

“You can think of it as using the minerals and rocks as hourglasses,” Prissel said. “Whenever an event happens – whether it’s a heating event, pressure change, or new pulse of magma – it’s like you’ve flipped the hourglass over. The sand starts falling as a reaction starts happening in the materials inside the volcano. When the volcano erupts, the reaction stops and the hourglass is frozen in time. This lets us see how much time elapsed between the initial event and the eruption.”
There are limitations on this method, and Prissel is quick to note that the determined diffusion rates in ilmenite often provide minimum time estimates. That is, this clock has an upper limit on how much time it can measure.
“The hourglass comparison works well because eventually all the sand falls through. That happens if the minerals are allowed to sit long enough for the reaction to go to completion or for everything to get back to equilibrium,” Prissel said. “If the products of the eruption are at equilibrium, that just tells us that the hourglass ran out. More time has passed, but we don’t know exactly how much.”
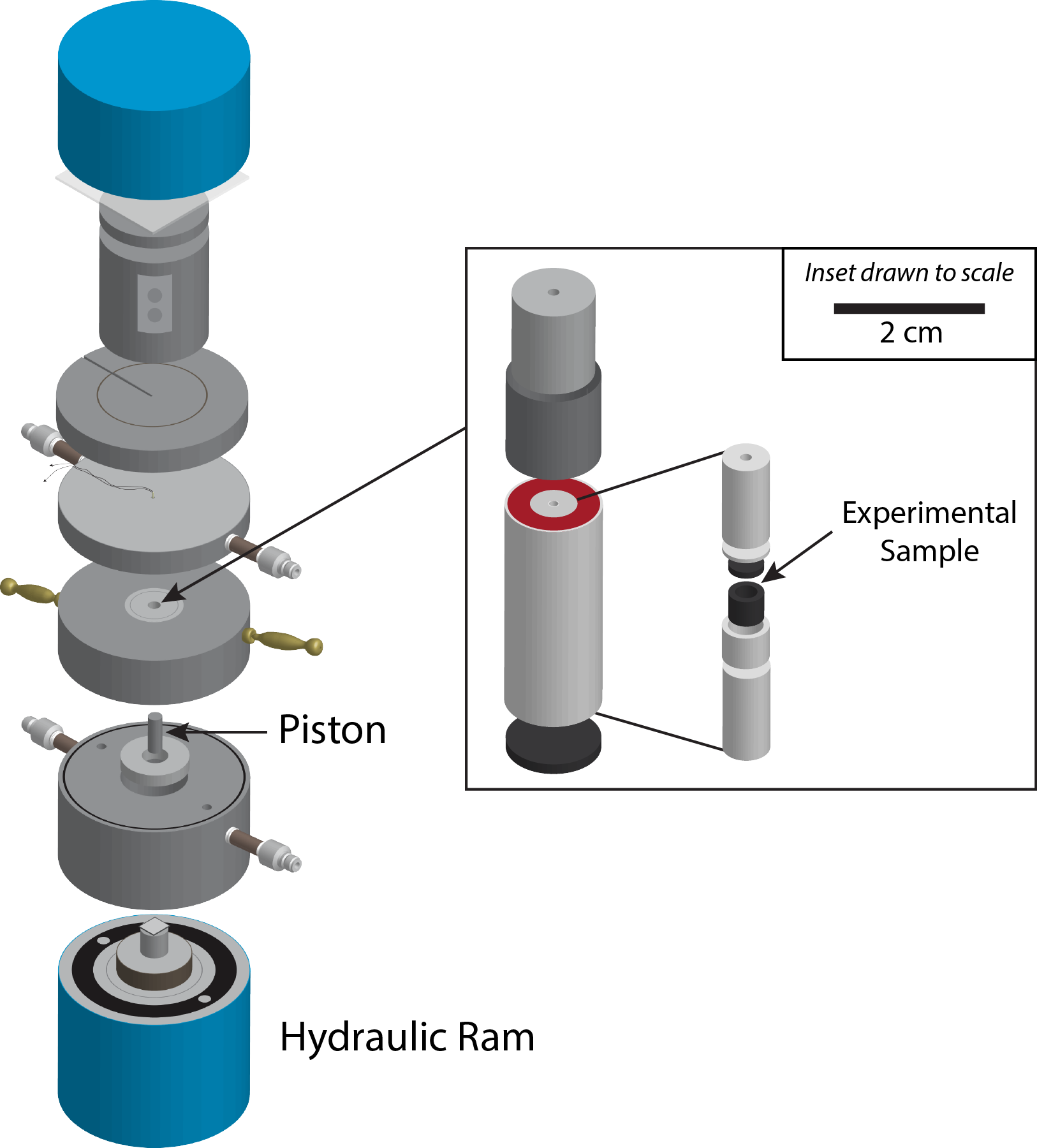
By creating mineral samples with varying concentrations of elements and allowing them to react under different conditions in the lab, Prissel was able to determine how fast certain chemical elements move within ilmenite. Experimentally determined rates can then be applied to natural samples and compared to existing data.
A surprising result came in the application of the new dataset to kimberlites, igneous rocks that form deep in Earth’s interior and in the long-distant past were brought to the surface by volcanic eruptions. Kimberlites are popularly best known for being diamond ores. Though there hasn’t been a kimberlite eruption in millions of years, Prissel et al.’s newly calibrated geospeedometer revealed that kimberlite magmas and large crystals known as megacrysts are interacting deep in the Earth for days or weeks prior to making the journey to the surface in mere hours. This finding could have implications for how geologists understand kimberlite eruptions and the enigmatic deep melting processes in Earth’s mantle.
Applications to modern volcanoes are no less exciting. This research shows that there are insights to be gained on volcanic activity over very short timescales, geologically speaking. The work also opens the door to additional similar studies on different elements. The team added that there are no limits on where scientists could use this method.
“There’s a lot of work still to be done. We’ve shown that these experiments can be done, and they should be done,” Krawczynski said. “The tools that are being applied here and the model development that Kelsey did is applicable to a lot of different systems. With other researchers doing these measurements on other minerals, we are now getting a much bigger picture as a community of these different timescales. As a field we’re all continuing to work on this problem and eventually want to be able to look at every different timescale with the proper tool.”
Acknowledgements
This work was supported by the Roger B. Chaffee fellowship from Washington University’s McDonnell Center for the Space Sciences, an earth and space sciences fellowship grant from NASA, and a petrology and geochemistry grant from the National Science Foundation.